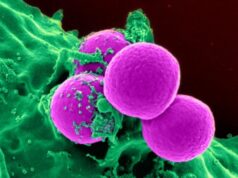
Toxic Shock Syndrome (TSS) is a severe circulatory and organ failure caused by bacterial toxins, usually triggered by bacteria from the Staphylococcus group. Researchers from MedUni Vienna’s Department of Clinical Pharmacology, in collaboration with the company Biomedizinische Forschungsgesellschaft mbH in Vienna, have now developed the world’s first safe and effective vaccine against this disease and successfully tested it in a Phase I trial. The promising results were recently published in the leading journal “The Lancet Infectious Diseases.”
This syndrome was first described in the 1980s. General symptoms of sepsis or blood poisoning occurred in young women who had used so-called “super tampons” during their periods. This is why the syndrome was also known as “tampon disease.” This subsequently led to the absorption capacity of tampons being regulated.
Staphylococci colonize nearly all of us, especially on our skin and mucous membranes. They are totally harmless to most people. “However, for people with weakened immune systems, they can cause serious diseases such as Toxic Shocks Syndrome,” explains Martha Eibl, director of Biomedizinische Forschungsgesellscaft mbH and former university professor at the Institute for Immunology of the medical faculty of the University of Vienna. This affects dialysis patients, the chronically sick, people with liver diseases and people recovering after heart operations. “Nevertheless, in 50% of cases the disease is associated with menstruation in young women,” says Bernd Jilma from MedUni Vienna’s Department of Clinical Pharmacology.
The vaccine, which has now been found to be safe and effective — and to have practically no side effects — in a clinical Phase I trial, and has been tested on 46 young men and women, was developed from a detoxified Staphylococcus toxin. The vaccine is injected into the skin and its effect is similar to that of a tetanus vaccination, says Jilma. “Immunization with such vaccines lasts for five years or more.” Once vaccinated, a person develops antibodies, which become active if the germs start to pose a threat. A blood test can show whether someone is short of antibodies. Risk groups could then be preventively vaccinated.
“We are well on the way to having a vaccine that prevents this series disease. However, it will still take some years before it is in clinical use,” explains Eibl. A Phase II trial with a larger test population has now started, in order to check the initial, promising results. “We are still looking for more volunteers,” says Jilma.
Find your dream job in the space industry. Check our Space Job Board »
Source: Medical University of Vienna
Journal: Lancet Infectious Diseases
Research Reference:
- M. Schwameis, B. Rappenser, C. Firbas, C. Gruener, N. Model, N. Stich, A. Roetzer, N. Buchtele, B. Jilma, M. Eibl.Safety, tolerability, and immunogenicity of a recombinant toxic shock syndrome toxin (rTSST)-1 variant vaccine: a randomised, double-blind, adjuvant-controlled, dose escalation first-in-man-trial. Lancet Infectious Diseases, June 2016 DOI: 10.1016.S1473-3099(16)30115-3