As cells bump into each other, forces cause them to move and shake, or even sometimes rupture.
“Cells are constantly generating forces and responding to them. They are being pulled on by their environment,” said Jonathan Winkelman, a postdoctoral researcher at the University of Chicago. Winkelman works in the lab of Margaret Gardel, professor in the Department of Physics and the Pritzker School of Molecular Engineering.
Unlike a rubber band that breaks when you stretch it too much, an overstretched cell initiates a response to repair itself. This phenomenon has been observed using microscopy, but the question of how the repair and adaptation process initiates inside the cells has remained unanswered until now.
In an innovative new study published Sept. 28 in the Proceedings of the National Academy of Sciences, Winkelman, along with several other University of Chicago researchers, demonstrated how a protein detects forces inside the cell and initiates a repair.
All cells have actin cytoskeletons—a network of filamentous protein that is essential for cell processes such as migration, growth, stretching, and more. “People had observed a protein called Zyxin in cells going to these stretched actin structures previously, but were not really sure how it was working or how widespread this function was,” said Winkelman.
Find your dream job in the space industry. Check our Space Job Board »
UChicago researchers discovered that animal proteins, including Zyxin and the single-cell fission yeast protein paxillin, were able to detect stressed materials in the actin cytoskeletons. Immediately after mechanical force was applied in a lab, the proteins assembled around where a repair is needed, and directly bonded to a stretched conformation of the actin filament.
Documenting their observation took a broad range of skills to develop the perfect assay to recreate this self-repair outside of the cell from purified components. Graduate student Caitlin Anderson, who co-first authored this work with Winkelman, screened proteins utilizing imagery and exquisitely sensitive assays she developed in a lab led by David Kovar, Professor of Molecular Genetics and Cell Biology.
Computer programs allowed them to comb through the human genome to isolate proteins likely involved in the process. A group called the LIM domain family of proteins appeared in the genome over 70 times, suggesting the importance of its conservation in human evolution.
Then in the Gardel lab, Winkelman applied a laser to act as an artificial way to mimic the damage done by the forces like stretching. They also added fluorescent tags to each of the LIM proteins and observed the process with high-powered microscopes. As soon as there was a tear or a rupture, the team observed that many of the 70+ LIM domain proteins encoded by the human genome rapidly detected the damage and bound to the afflicted sites. It was clear that LIM force sensitivity was widespread, and had been copied and pasted into dozens of various proteins by evolution, the scientists said.
“We were looking at this group of proteins to mount this detection and repair response in highly complex cells that contain thousands of different types of proteins,” said Winkelman. “However, to really understand this process, we needed to purify the essential components and rebuild the whole process outside of the cells.”
Anderson used a technique called Total Internal Reflection Fluorescence microscopy and a complicated process to create a very pure sample of just the protein they needed—something that had never been done before.
They also discovered this force-sensing via LIM is seen in both yeasts and mammals, suggesting it is an ancient function that evolution protected and propogated. This highly conserved, ancient mechanism is likely to be used by an array of other processes to sense forces within cells.
“Cellular force-sensing via LIM domains could inform many other processes besides self-repair, such as controlling stem cell fate, cell proliferation, or cell migration, and many more diverse signaling pathways that need further exploration,” said Gardel. “What Jon and Caitlin found is that there are many of these proteins that share this domain.”
“The work furthers our understanding of fundamental science—how cells detect and process mechanical signals, how diverse mechanical pathways are regulated in epithelial cells and adherent tissues,” said Gardel. “But there are also applications to building soft, responsive materials in a nonbiological context that have a same recognition process.”
Provided by: University of Chicago
More information: Jonathan D. Winkelman et al. Evolutionarily diverse LIM domain-containing proteins bind stressed actin filaments through a conserved mechanism. Proceedings of the National Academy of Sciences (2020). DOI: 10.1101/2020.03.06.980649
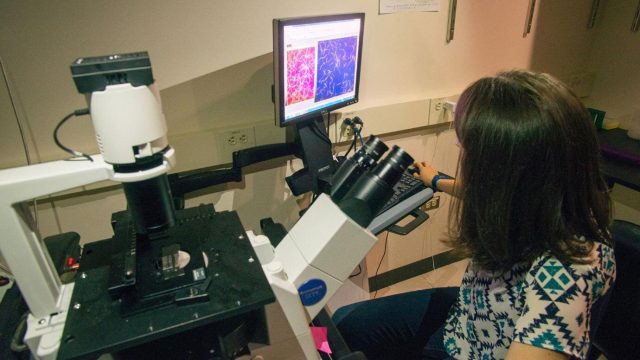
Image: University of Chicago graduate student Caitlin Anderson and a team of researchers demonstrated how a protein detects forces inside the cell and initiates a repair. Above, Anderson checks images from the microscope.
Credit: Tricia Koning/OGPA/UChicago Biosciences