Bacteria have multiple strategies to survive antibiotics: developing genetic resistance to the drugs; delaying their growth; or hiding in protective biofilms. New results from researchers at Princeton and California State University-Northridge (CSUN) have shed light on yet another approach: self-sacrifice.
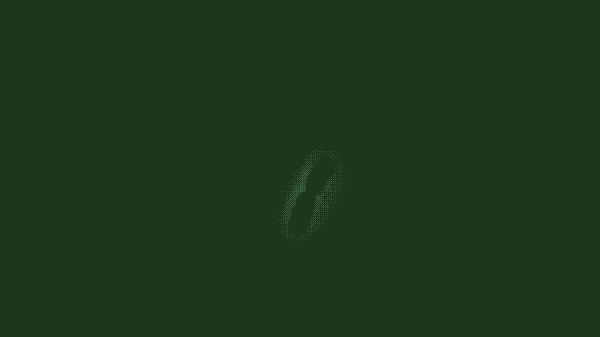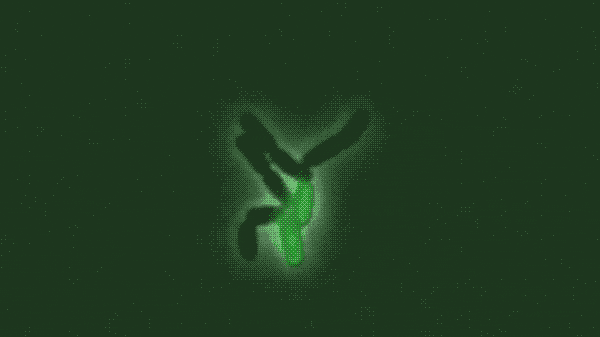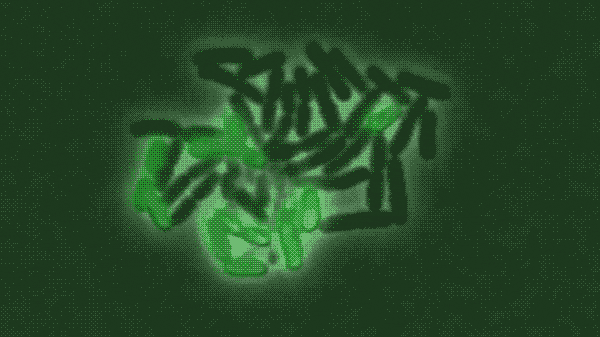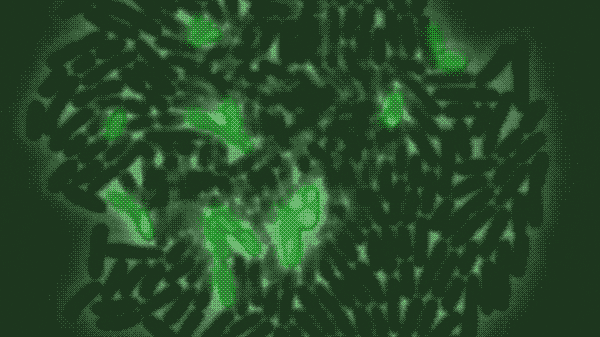
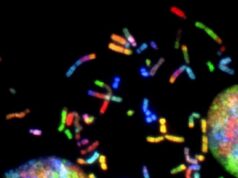
In a population of E. coli bacteria treated with a particular antimicrobial molecule, the researchers found, some dying cells absorbed large amounts of the antibiotic, allowing their neighbors to survive and continue growing. The researchers created a modified, green fluorescent version of the antibiotic of interest, a peptide molecule known as LL37 that is naturally produced by human skin, airways and other organs that frequently contact bacteria from the outside world. Tracking the glowing molecule’s movements through a population of bacteria, as shown in the figure above, revealed that the antibiotic was accumulating in a subset of dying cells.
Andrej Košmrlj, an assistant professor of mechanical and aerospace engineering at Princeton, collaborated with the CSUN team to develop a mathematical model to more fully explain the phenomenon and aid further investigations.
The model describes the dynamics of bacterial populations facing different concentrations of the antimicrobial, showing how dead cells sequester the dangerous molecule and predicting the delayed growth of surviving cells—calculations borne out by experiments in the laboratory of Sattar Taheri-Araghi, an assistant professor of physics at CSUN and co-senior author of the study along with Košmrlj.
“The model provided a physical explanation for how this actually works,” said Košmrlj. “We had a surprising observation that the critical inhibitory concentration of antimicrobial peptides depends on the number of bacteria, and our model was able to explain why this happens.”
Find your dream job in the space industry. Check our Space Job Board »
Despite this new understanding, questions remain about what is happening at the molecular level, said Taheri-Araghi. “This research opens the doors to a lot of questions that were never asked before. Our findings have profound implications for the evolution of bacteria—which have been around for billions of years—as well as in medicine for the design and administration of novel antibiotics.”
The researchers reported their results in a paper published Dec. 18, 2018, in eLife.
Provided by:
Princeton University
More information:
Mehdi Snoussi et al. Heterogeneous absorption of antimicrobial peptide LL37 in Escherichia coli cells enhances population survivability. eLife (2018). DOI: 10.7554/eLife.38174
Image:
In a population of E. coli bacteria treated with a particular antimicrobial molecule (labeled in green), some dying cells absorb large amounts of the antibiotic, allowing their neighbors to survive and continue growing. This figure shows a time-lapse of bacterial growth over four hours.
Credit: Beatrice Trinidad