Researchers have discovered a network of channels inside bacterial communities which could be used to kill bacteria more quickly by ‘tricking’ them into transporting drugs.
The communities—called biofilms—are involved in up to 80% of persistent human infections and cannot be killed easily by antibiotics.
Researchers in the Department of Physics and the Strathclyde Institute for Pharmacy and Biomedical Sciences at the University of Strathclyde have found that when the bacterium E. coli is living as a biofilm, it forms channels to help transport nutrients around its community.
They discovered these channels to transport nutrients into the biofilm could also be exploited to design new methods for delivering drugs to destroy the biofilm ‘fortresses’ and killing the bacteria within it.
Dr. Liam Rooney, who carried out the research while a Ph.D. student at the University of Strathclyde and is lead author on the paper, published in Nature‘s International Society of Microbial Ecology Journal, said, “What we discovered is a network of nutrient-transporting channels that are formed when bacteria grow in large communities, and this could be used to kill bacteria more quickly by tricking the bacteria into transporting drugs through the channels instead of food.”
Find your dream job in the space industry. Check our Space Job Board »
He explained, “It’s like a bacterial Death Star, with the biofilms able to be blown up from the inside by targeting the channel systems with drugs. The structure of large bacterial communities is complex and poorly understood but the discovery of these channel systems in E. coli biofilms is important because it tells us how bacteria in biofilms can move nutrients throughout their community. We can then use this knowledge to design better drugs and treatment strategies to lower their impact in the clinic and in industry.”
The two-year study, funded by the Medical Research Council and Natural Environment Research Council, used an advanced light microscope called the Mesolens, formed via a collaboration between researchers at Strathclyde and the Laboratory of Molecular Biology. It visualized how billions of individual bacteria arranged to form complex multi-millimeter scale bacterial communities.
Dr. Rooney, who is now a post-doctoral researcher at Edinburgh’s Heriot-Watt University, added: “By using the Mesolens we have been able to shine a light on biofilm architecture and visualize them like never before. As well as helping to understand how bacteria form large structures, our research shows how their role in transport can be exploited to lower the burden of biofilms in the clinic.”
Protective shells
As well as acting as protective shells which keep out potential treatments, biofilms are their most dangerous when they form during infections or form on sutures and catheters or ventilators, increasing the risks of the patient developing an infection.
Dr. Rooney said that the findings could ‘revolutionize’ the way infections are treated and added: “The channels take the nutrients from underneath to transport them through the biofilm, whereas traditionally antibiotic treatment would be from above the biofilm.
“These biofilms are like domes, which we would normally try to remove by applying antibiotics to their surface, but the problem is that antibiotics aren’t really penetrating into it. This then leads to persistence of the infection and then development of antimicrobial resistance, which is a major public health issue. Because we’ve found a secret route into the biofilm from underneath, then potentially we can get the drugs in under the dome to kill the bacteria quicker and more effectively.”
He continued, “Nobody has been able to show how food and nutrients could go so deep inside a biofilm because these channel structures haven’t been observed before. There may also be a lower chance of a relapse in infection and it could revolutionize the way infections are treated as there is potential to develop methods to try and access the channels by delivering the drugs in a different way.”
Healthcare-associated infections are estimated to cost the NHS around £1 billion a year, while the cost of health care for patients with resistant infections is higher due to longer duration of illness, additional tests and use of more expensive drugs.
Cystic fibrosis
Biofilms are also a major problem in Cystic Fibrosis patients, as there are biofilms which literally clog up the lungs which can exacerbate the condition.
The team are looking at a number of follow-up projects and say the next steps are to understand the formation and conditions in which these structures form.
Co-author, Professor Gail McConnell from Strathclyde said: “This could change the way that pharmaceutical companies approach treatment strategies and potentially the long term health benefits for society are huge. There are some assumptions about how these biofilms form which we’ve shown are wrong and we hope this will lead to great change.”
Provided by: University of Strathclyde, Glasgow
More information: Liam M. Rooney et al. Intra-colony channels in E. coli function as a nutrient uptake system. The ISME Journal (2020). DOI: 10.1038/s41396-020-0700-9
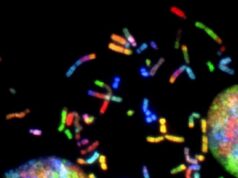
Image: Biofilm.
Credit: University of Strathclyde, Glasgow