Helical shapes are very familiar in the natural world and, at the molecular level, of DNA, the very blueprint of life itself.
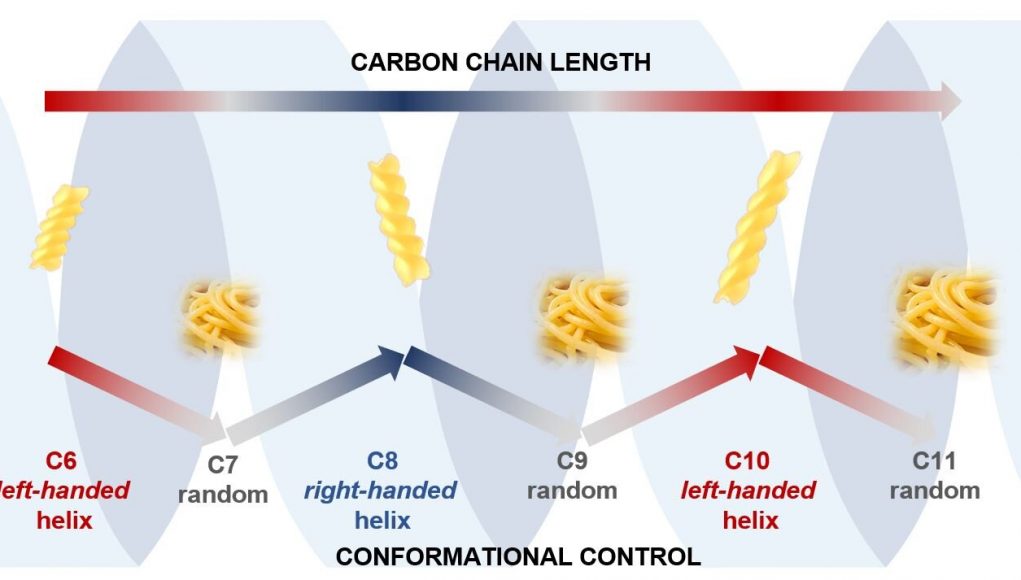
Scientists at the University of Bristol have now found that carbon chains can also adopt helical shapes, but, unlike DNA, the shape is dependent on how many atoms there are in the chain, with chains having even numbers of carbon atoms adopting helical, fusilli-like shapes and chains with odd numbers of carbon atoms adopting floppy, spaghetti-like shapes.
The difference, say the research team, between order and chaos is a single carbon atom. Their study is published today in the journal Nature Chemistry.
Carbon chains are like spaghetti—they are rather floppy and adopt a set of random and constantly changing shapes.
The Bristol team, from the University’s School of Chemistry, showed that by judicious insertion of methyl substituents along carbon chains they could control their shape so that they adopted well-defined linear (penne) or helical (fusilli) conformations.
Find your dream job in the space industry. Check our Space Job Board »
The helical conformations can adopt either right or left-handed helices and the team were interested to know what controlled which helix was formed.
Lead author, Professor Varinder Aggarwal, said: “We were astonished to find that the length of the carbon chain (number of carbon atoms) controlled whether the right or left-handed helix formed.
“Even more surprising was that carbon chains with even numbers of atoms formed well-defined helical structures (fusilli) but odd numbered carbon chains were much floppier and more random in shape (spaghetti).
“The change in properties of a homologous series of molecules caused by the single addition of an extra carbon atom is extremely rare—here it results in the difference between order and chaos.”
This type of odd-even effect has been observed in some bulk properties, such as in carpets of alkanethiols on a gold surface, but such behaviours in solution are not well recognised or understood.
Through computation and measurement of molecular properties, Professor Aggarwal and his team have been able to fully understand the origin of this odd-even effect which is controlled by the end groups.
When the end groups both promote the same sense of helicity, an ordered structure is obtained, but when each end promotes an opposite helix, chaotic structures are obtained.
For future technological applications, these fundamental findings will guide the design of molecules with desirable conformational, and physical properties.
Carbon chains with an even number of atoms will lead to molecules with well-defined helical shapes for their application as non-switchable rigid materials or as scaffolds for the presentation of molecular recognition elements.
Helices are a fundamental structure in biological molecules (DNA, proteins) and it is intriguing to imagine the analogies to molecules of the sort described in the study.
Professor Aggarwal added: “Carbon chains with an odd number of atoms were found to adopt floppier and more random shape.
“We are now studying whether the shape of these chains can in fact be controlled by manipulating the groups at the ends of the chain. This may enable us to switch from one screw-sense to another for applications in responsive materials.”
Provided by: University of Bristol
More information: Johan A. Pradeilles et al. Odd–even alternations in helical propensity of a homologous series of hydrocarbons. Nature Chemistry (2020). DOI: 10.1038/s41557-020-0429-0
Image: The image shows how the conformation (shape) of our carbon chains alternate between ordered and chaotic structures as the carbon chain alternates between having even and odd numbers of atoms.
Credit: University of Bristol