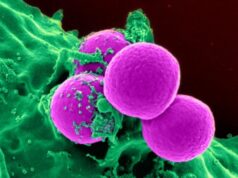
Bacteria (green) inside human pancreatic cancer cells (AsPC-1 cells). The cells’ nuclei are stained blue while their cytoplasm is stained orange.
Credit: Weizmann Institute of Science
To the reasons that chemotherapy sometimes does not work, we can now add one more: bacteria. In a study published in Science, researchers describe findings that certain bacteria can be found inside human pancreatic tumors. The findings further showed that some of these bacteria contain an enzyme that inactivates a common drug used to treat various cancers, including pancreatic cancer. Working with mouse models of cancer, they demonstrated how treatment with antibiotics on top of chemotherapy may be significantly superior to treatment with chemotherapy alone.
The bacteria the group found, explains Straussman, live within the tumors, and even within the tumor cells. “Because the topic is so new, we first used different methods to prove that there really were bacteria inside the tumors. Then we decided to look at the effect that these bacteria might have on chemotherapy.”
The researchers isolated bacteria from the tumors of pancreatic cancer patients and tested how they affect the sensitivity of pancreatic cancer cells to gemcitabine, a chemotherapy drug. Indeed, some of those bacteria kept the drug from working. Further investigation showed that these bacteria metabolize the drug, making it ineffective. The researchers were able to find the bacterial gene responsible for this, a gene called cytidine deaminase (CDD). They demonstrated that CDD comes in two forms — a long and a short form. Only bacteria with the long form of the CDD gene could inactivate gemcitabine. The drug had no apparent effect on the bacteria.
The group examined over 100 human pancreatic tumors to show that these particular bacteria with long CDD do live in the patient’s pancreatic tumors. They also used multiple methods to visualize the bacteria inside human pancreatic tumors. This is crucial, since bacterial contamination is a real issue for lab studies.
Oddly enough, it was an earlier incidence of bacterial contamination that led Straussman and his team to this present study. He and his group had been looking for evidence that normal cells in the cancer’s environment contribute to chemotherapy resistance. While testing the effect of many normal, non-cancerous, human cells on the sensitivity of cancer cells to chemotherapy, they found a specific sample of normal human skin cells that rendered pancreatic cancer cells resistant to gemcitabine. Tracking down the cause led the team to bacteria that had accidently contaminated these skin cells. “We nearly threw it away,” says Straussman, “but then we decided to follow it up, instead.” After revealing how these bacteria degraded the drug, he began to wonder if other bacteria might have a similar mechanism for inactivating the drug, and whether such bacteria might be found inside human tumors.
In the present study, further experiments in mouse models of cancer were done with two groups of bacteria: those containing the long form of the CDD gene and those in which the gene had been knocked out. Only the group with the CDD gene intact exhibited resistance when the drug was given to the mice. After treatment with antibiotics, this group also responded to the chemotherapy drug.
Many questions remain, and Straussman and his group are now asking whether bacteria may be found in other cancer types and, if so, what effects they might have on the cancer and its sensitivity to other anti-cancer drugs including a novel family of immune-mediated anti-cancer drugs.
Dr. Ravid Straussman’s research is supported by the Dr. Dvora and Haim Teitelbaum Endowment Fund; the Hymen T. Milgrom Trust donation fund; the Rising Tide Foundation; and Mr. and Mrs. Andrew R. Morse. Dr. Straussman is the incumbent of the Roel C. Buck Career Development Chair.
The Weizmann Institute of Science in Rehovot, Israel, is one of the world’s top-ranking multidisciplinary research institutions. Noted for its wide-ranging exploration of the natural and exact sciences, the Institute is home to scientists, students, technicians and supporting staff. Institute research efforts include the search for new ways of fighting disease and hunger, examining leading questions in mathematics and computer science, probing the physics of matter and the universe, creating novel materials and developing new strategies for protecting the environment.
Story Source: Materials provided byUniversity of Chicago Original written by Whitney Clavin.Note: Content may be edited for style and length.
Journal Reference:
Leore T. Geller, Michal Barzily-Rokni, Tal Danino, Oliver H. Jonas, Noam Shental, Deborah Nejman, Nancy Gavert, Yaara Zwang, Zachary A. Cooper, Kevin Shee, Christoph A. Thaiss, Alexandre Reuben, Jonathan Livny, Roi Avraham, Dennie T. Frederick, Matteo Ligorio, Kelly Chatman, Stephen E. Johnston, Carrie M. Mosher, Alexander Brandis, Garold Fuks, Candice Gurbatri, Vancheswaran Gopalakrishnan, Michael Kim, Mark W. Hurd, Matthew Katz, Jason Fleming, Anirban Maitra, David A. Smith, Matt Skalak, Jeffrey Bu, Monia Michaud, Sunia A. Trauger, Iris Barshack, Talia Golan, Judith Sandbank, Keith T. Flaherty, Anna Mandinova, Wendy S. Garrett, Sarah P. Thayer, Cristina R. Ferrone, Curtis Huttenhower, Sangeeta N. Bhatia, Dirk Gevers, Jennifer A. Wargo, Todd R. Golub, Ravid Straussman. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science, 2017; 357 (6356): 1156 DOI: 10.1126/science.aah5043